
Founded by Pavan Kalyan, Nirmit Arora & Gunin Gupta
Hey everyone 👋 Meet Pavan (PK), Gunin and Nirmit. The co-founders of Ritivel.
Ask: Can you introduce the founders to regulatory affairs leaders or CISOs at pharma companies? Contact them via email here.
The founders are engineers who previously built AI copilots at Microsoft Research. They saw firsthand that they could create better, more specialized copilots for complex workflows.
The team started by selling a generic copilot to process-heavy industries like life sciences to understand their regulatory workflows. After 50+ conversations with pharma professionals, they discovered the critical bottleneck in regulatory submissions and built Ritivel to solve it.

Every month a drug is delayed from reaching the market costs pharma companies ~$45M in lost revenue and more importantly, delays treatment for thousands of patients who need it.
Yet regulatory teams are stuck in a painful, months-long process to create FDA submissions like CTDs, CSRs, INDs, and BLAs:
Ritivel deploys AI agents that transform how regulatory teams work:
Draft the most common document types like CTDs, CSRs, INDs, and BLAs in minutes ✍️ Ritivel agents generate comprehensive first drafts directly in Microsoft Word, following FDA formatting requirements and pulling from your existing clinical data and prior submissions. Every data point includes citations linked back to the original source, so reviewers can verify with one click.
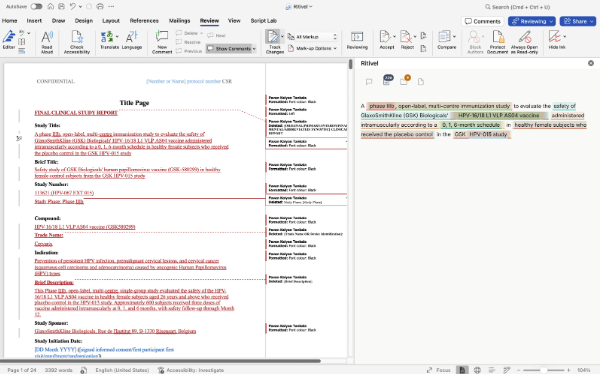
Intelligent document retrieval 🔗 Connected to SharePoint and Veeva, Ritivel agents automatically source the right documents from across your organization: no more digging through folders or chasing down files.
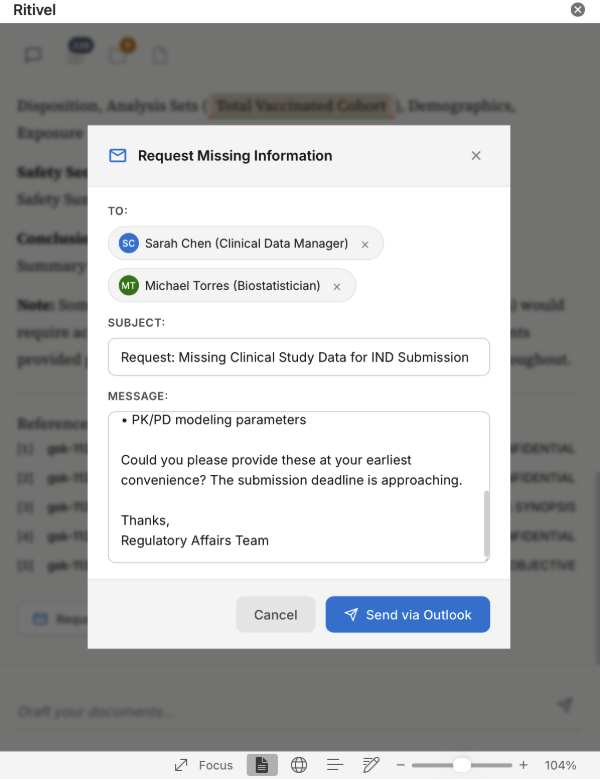
Automated follow-ups 📧 Integrated with Outlook, Ritivel agents handle reminder emails and follow-ups automatically, so you're not stuck chasing colleagues for missing documents.
Accelerated project management 🚀 By automating the document gathering and drafting loop, teams can focus on review and refinement rather than administrative overhead.